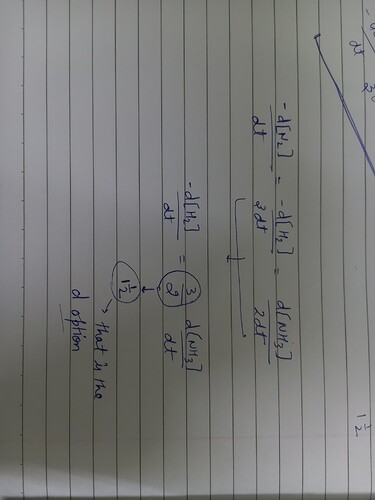
Please explain how option (d) is the answer and not option (b)?
- The rate of formation of ammonia by the reaction: N_{2} + 3H_{2} → 2NH_{3} expressed as (d[NH_{3}])/(dt) = 2.5 * 10 ^ 4 * mol * I ^ 1 * s ^ - 1
The rate of consumption expressed in terms of H_{2} as (- d * [H_{2}])/(dt) will be
a) Double
b) Three times
c) Same
d) One and a half time of that expressed in terms of N*H_{3}