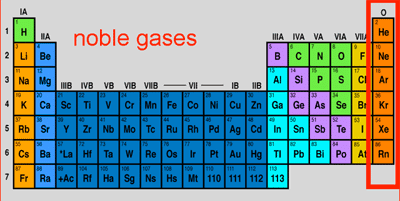
Why noble gas has large atomic radius
For the noble gases these include low reactivity, low boiling points, high ionization, complete valence shell, very low electronegativities, and they are colorless as well as odorless
1 Like
Noble gasses have comparatively large atomic sizes because they have vander waals’ radii only which are expected to have larger magnitude where as other memebers of a period have either covalent or metallic radii which are less in magnitude.
1 Like