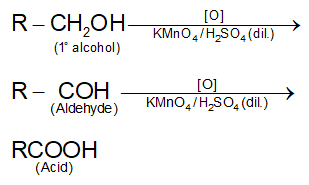CHEMISTRY FORMULA FOR CLASS 11 CHAPTER- SOME BASIC CONCEPT OF CHEMISTRY
Chemistry Formulas
- Matter is substance which has mass and occupies space, e.g., water, air, box, table, etc.
- State of Matter: Solid, liquid and gaseous state represent three different states of mater. Gaseous mater at very high temperatures contains gaseous ions and free electron and is referred to as the plasma state, i.e., fourth state of matter.
- Homogenous: These have uniform. composition throughout, e.g., elements and compounds. Mixture can also be homogeneous.
- Heterogeneous: These do not have uniform Entrance composition, e.g., mixture of iron fillings and sulphur powder.
- Element: It is a substance which cannot be further divided by chemical methods. It is a pure substance and consists of only one kind of atoms. They are metals, non-metals and metalloids (resemble both metal and non metal), e.g., F, Al, Ni, Au, Ag are metals. C, S, N2, I2 are non-metals. B. Si, As, Sb are metalloids. They are pure substances.
- Compound. It is a pure substance made up of two or more elements combined together in fixed ratio, e.g., NaCl, FeS, CuSO4, H2O, etc. Its components cannot be separated by simple physical methods.
- Organic compounds: They are compounds containing carbon and few other elements like H, N, S, halogens, phosphorus, oxygen etc. e.g., sugar, urea, glucose, etc.
- Inorganic compounds: They are made up of two or more elements and obtained form minerals and ores, e.g., NaCl, KCl, CaCO3, etc.
- Mixture: It consists of two or more elements or compounds in any ratio. Its components can be separated by simple physical methods. It can be homogeneous (e.g., solution of salt in water, sugar in water). It can be heterogeneous. e.g., smoke dust particles in air, air, etc. Homogeneous mixture is also called solution.
- Separation of Mixtures: Mixtures are separated into its components depending upon the property of components in which they differ.
- Filtration: It is used to separate suspended (insoluble) component from a soluble component which passes into the filtrate and the insoluble component is left behind on the filter paper. Soluble component can be obtained by evaporation.
- Simple Distillation: It is used for separation of miscible liquids which differ in their boiling points appreciably, e.g., benzene and toluene can be separated by simple distillation.
- Fractional Distillation: It is used for separation of miscible liquids which differ in their boiling points appreciably, e.g., benzene and toluene can be separated by simple distillation.
- Molecules: They are identifiable units of matter consisting of two or more atoms of the same element or of different elements combined in a definite ratio.
- Atomicity: It is defined as the number of atoms present in a molecule.
- Monoatomic: They consist of only one atom, e.g., He, Ne, Ar, Kr, Xe, Na, K, etc.
- Diatomic: They consist of two atoms, e.g., H2, Br2, Cl2, F2, I2, N2, O2, HCl, HBr, etc.
- Triatomic: They consist of three atoms, e.g., O3, H2O, SO2, CO2 etc.
- Tetra-atomic: They consist of four atoms, e.g., P4, SO3, NH3 etc.
- Penta-atomic: It contains five atoms, e.g., CH4, CCl4, SiCl4 etc.
- Hexa-atomic: It contains six atoms, e.g., C2H4, H2SO3.
- Hepta-atomic: It contains seven atoms, e.g., H2SO4.
- Octa-atomic: It contains eight atoms, e.g., S8 is octa-atomic molecule.
Mole Concept
A mole is the amount of a substance that contains as many atoms, molecules, ions or other particles as there are atoms in 0.012 kg (or 12g) of the carbon-12 isotope. The mole of a substance always contains same number of particles, whatever the substance may be.
Laws of Chemical Combinations
- Law of Conservation of Mass (discovered by Lavosier): Matter can neither be created nor be destroyed. The total mass of reactants must be equal to total mass of products. For example C + 02 → CO2
12g + 32g = 44g
That is why we balance each and every chemical reaction.
- Law of Constant Composition (discovered by Proust): A compound prepared by any method contains the same elements in the fixed ratio by mass, e.g., H2O prepared by any method contains hydrogen and oxygen in the fixed ratio 1 : 8 by mass.
- Law of Multiple Proportion (discovered by Dalton): When two elements combine to form two or more chemical compounds, then the weights of one of the elements which combine with the fixed weight of another, bear a simple ratio to one another, e.g., carbon reacts with oxygen to form carbon monoxide and carbon dioxide.
In CO, 12 parts by weight of carbon combine with 16 parts by weight of oxygen.
In CO2, 12 parts by weight of carbon combine with 32 parts by weight of oxygen.
The ratio between different weights of oxygen that combine with 12 parts (fixed weight) of carbon is 16 : 32, i.e., 1 : 2
Law of Reciprocal Proportion (discovered by Richter)
- When two elements combine separately with a fixed mass of third element, then the ratio of their masses in which they combine will be either same or simple multiple of the ratio in which they combine with each other, e.g., C and O2 react with S to form CS2 and SO2 whereas carbon reacts with O2 to form CO2
- 32 parts by weight of S combine with 32 parts by weight of oxygen in SO2
- 32 parts by weight of S combine with 6 parts by weight of carbon in CS2.
- The ratio 6 : 32 which is multiple of 12 : 32 in CO2.
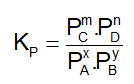
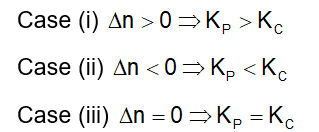
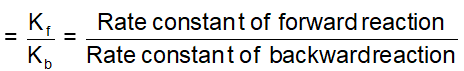
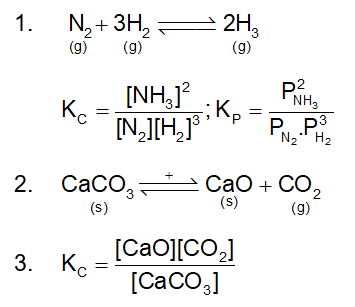
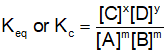
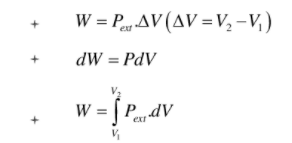
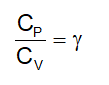
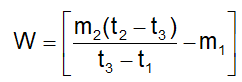
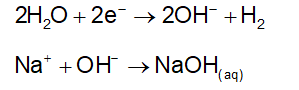
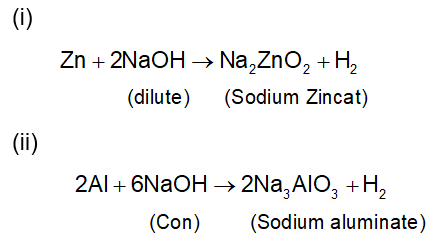
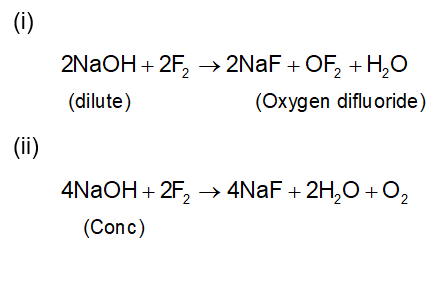
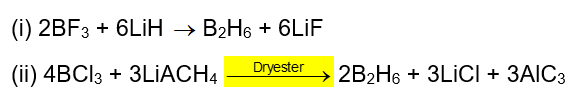
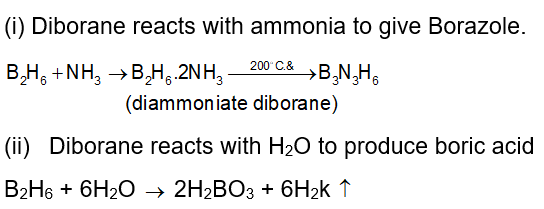
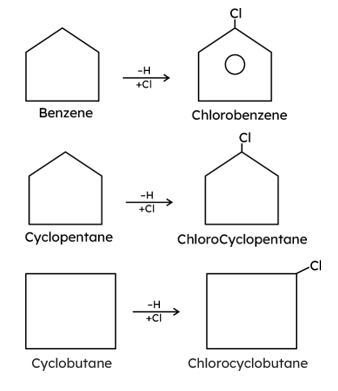
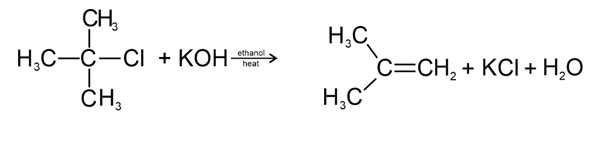
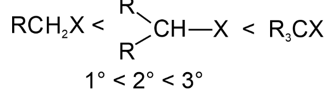
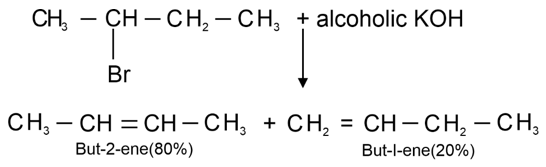
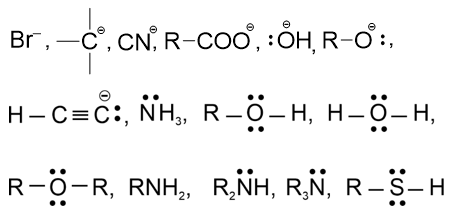
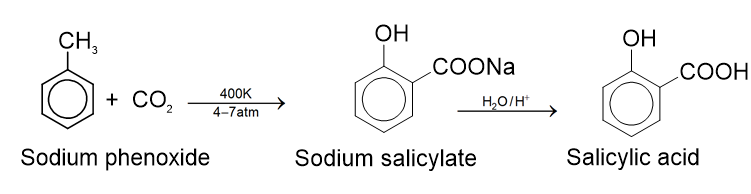
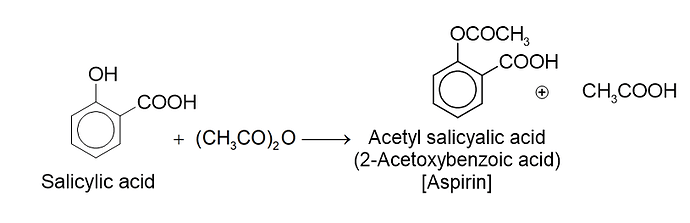
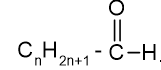 . Their functional group is
. Their functional group is 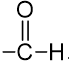 Some of them are unpleasant smelling compounds. They contain carbonyl group attached with hydrogen. They are good reducing agents, e.g., HCHO (formaldehyde), CH3CHO (Acetaldehyde), etc.
Some of them are unpleasant smelling compounds. They contain carbonyl group attached with hydrogen. They are good reducing agents, e.g., HCHO (formaldehyde), CH3CHO (Acetaldehyde), etc.